Our Mission
The mission of the Coalition for Clinical Trials Awareness is to advocate for the creation of a federally sponsored public awareness campaign to increase the public’s understanding of the benefits of clinical trials.
The Challenge
Lack of Patient Awareness = Lack of Participation = Delays & Higher Costs
Clinical trials are under-enrolled and difficult to fill. A recent study conducted by the Tufts Center for the Study of Drug Development involving 150 clinical trials and nearly 16,000 study sites found that 11% of sites fail to enroll even one patient and 37% do not meet their enrollment goals.
In 2003, an article published in the Journal of Clinical Oncology entitled Public Attitudes Toward Participation in Cancer Clinical Trials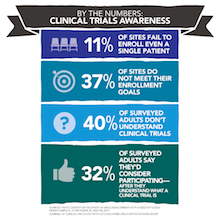 discussed the results of survey of 1000 healthy adults which showed that 40% of the survey participants did not understand the idea of a clinical trial. However 32% indicated that they would be very willing to participate in a cancer clinical trial if asked to do so.
discussed the results of survey of 1000 healthy adults which showed that 40% of the survey participants did not understand the idea of a clinical trial. However 32% indicated that they would be very willing to participate in a cancer clinical trial if asked to do so.
Another recent survey found most Americans had little or no knowledge of clinical trials and less than 10% reported knowing “a lot”. Of those recently exposed to information about clinical trials, most reported a more favorable perception as a result. Of the approximately half of the respondents who said they were unlikely to participate in a clinical trial, they cited concerns about treatment effectiveness or side effects (44%), mistrust of research organizations (20%), desire to wait for treatment approval (18%), and cost (15%).
Institute of Medicine (IOM) studies recognize the seriousness of the clinical trial enrollment problem. In 2012, the IOM published a summary of their workshop entitled Public Engagement and Clinical Trials, in which they identify “the increasing difficulty of recruiting and retaining an appropriate human subject population for specific clinical trials” as a primary problem.
The IOM report identified numerous barriers to clinical trial participation that can be overcome with a public awareness campaign. The barriers include lack of awareness that clinical trials are available (on the parts of both patients and physicians), lack of awareness of the societal benefits of clinical trials and difficulty maintaining neutrality with regard to treatment preference (for example, patients or physicians prefer one treatment arm over another).
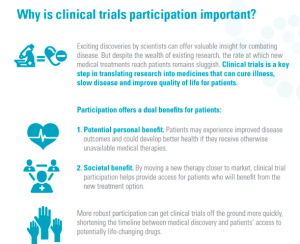
Increased Awareness = Greater Participation = Patient Access to New Therapies
These studies and reports confirm what researchers have long known… that there is a great need to promote the societal, as opposed to individual, benefits of clinical trials. While an individual patient may or may not personally benefit from clinical trial participation, broad participation benefits society as a whole by identifying new treatments that represent advances in efficacy and safety from those that do not. Providing education and information about the benefits of clinical trials leads to more patients being willing to consider participating in a trial.


Comments are closed.